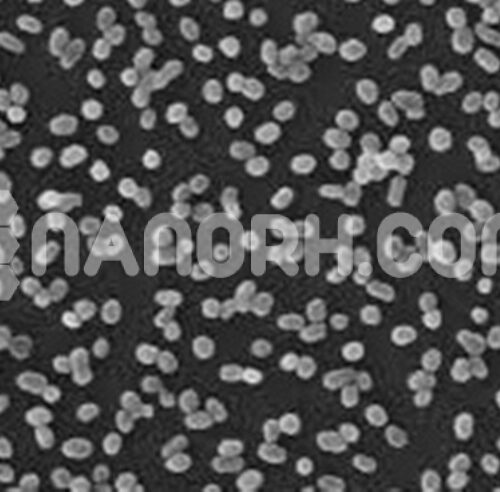
| Zinc Stannate Powder | |
| Product No | NRE-11294 |
| CAS No. | 12036-37-1 |
| Formula | ZnSnO3 |
| APS | <40 µm (Can be Customized) |
| Purity | 99.9% |
| Color | White |
| Molecular Weight | 232.15 g/mol |
| Density | 3.9 g/cm3 |
| Melting Point | 570°C |
| Boiling Point | NA |
Zinc Stannate Powder
Introduction:
Zinc Stannate powder is an inorganic compound composed of zinc (Zn), tin (Sn), and oxygen (O). It is a mixed metal oxide that combines zinc oxide (ZnO) and tin oxide (SnO₂) in a specific stoichiometric ratio. Zinc stannate is a white, crystalline powder that has a variety of applications due to its unique electronic and optical properties. It is known for being an excellent semiconductor and having useful catalytic and luminescent characteristics.
Applications
Transparent Conducting Oxides (TCOs) for Solar Cells:
Zinc stannate is a transparent conducting oxide (TCO) that is used in the development of thin-film solar cells. TCOs are essential materials in solar energy applications because they allow light to pass through while also conducting electricity. Zinc stannate, with its high transparency and electrical conductivity, is ideal for use as a front contact layer in photovoltaic cells.
Zinc stannate is particularly useful in solar cell manufacturing because it can be deposited onto a substrate in thin layers, providing an effective conducting layer that doesn’t absorb too much light, allowing more light to reach the photovoltaic material underneath.
Electronics and Semiconductors:
Zinc stannate is an attractive material for use in electronics due to its semiconducting properties. It can be used in thin-film transistors (TFTs), light-emitting diodes (LEDs), and other semiconductor devices. Zinc stannate thin films are often employed in the fabrication of optoelectronic devices, where both electrical conductivity and optical transparency are required.
Catalysis:
Zinc stannate exhibits catalytic properties and is used in catalytic applications such as oxidation reactions, hydrogenation, and dehydrogenation processes. Its surface area and chemical stability make it a valuable catalyst in various chemical reactions, particularly in the production of fine chemicals and petrochemical processing.
Zinc stannate is also employed as a catalyst in environmentally friendly processes where it can help reduce the emissions of harmful gases or serve as a catalyst for selective oxidations of organic compounds in industrial applications.