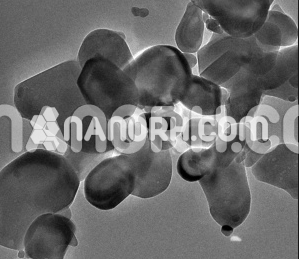
| Vanadium III Sulfate Nanopowder | |
| Product No | NRE-5255 |
| CAS No. | 13701-70-7 |
| Formula | V2(SO4)3 |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | Yellow |
| Molecular Weight | 390.074 g/mol |
| Density | NA |
| Melting Point | 400°C |
| Boiling Point | NA |
Vanadium III Sulfate
Applications:
Vanadium Redox Flow Batteries (VRFBs)
Vanadium III sulfate plays a crucial role in the development of vanadium redox flow batteries (VRFBs), which are used for large-scale energy storage. In these batteries, vanadium ions in different oxidation states (V²⁺, V³⁺, V⁴⁺, and V⁵⁺) are stored in electrolyte solutions and undergo oxidation-reduction reactions to store and release energy.
Electrolyte Production: vanadium III sulfate is used as a precursor to synthesize the vanadium electrolyte solutions in VRFBs. The vanadium III ions in the sulfate solution participate in the electrochemical reactions within the battery, allowing for efficient energy storage and conversion.
Energy Density and Efficiency: The use of vanadium compounds, particularly V₂(SO₄)₃, helps increase the energy density and operational efficiency of VRFBs, making them an essential technology for renewable energy storage, especially in grid-scale applications.
Production of Vanadium Alloys and Steel
Vanadium is widely used in the production of alloys, particularly in high-strength steel and titanium alloys, due to its ability to improve the strength, toughness, and corrosion resistance of metals.
Steel Industry: Vanadium III sulfate is used as a precursor in the production of vanadium metal, which is then alloyed with steel. Vanadium alloys are commonly used in the manufacture of high-strength low-alloy (HSLA) steels used in construction, tools, automotive components, and military applications. The addition of vanadium enhances the steel’s hardness, resilience, and wear resistance.
Titanium Alloys: Vanadium is also used in titanium alloys, which are important in industries like aerospace, medical implants, and high-performance machinery.
Catalysis in Chemical Reactions
V2(SO4)3 is involved in various catalytic processes, particularly in reactions that require vanadium in its lower oxidation states.
Catalysts in the Petrochemical Industry: V₂(SO₄)₃ is used as a precursor for vanadium catalysts in petroleum refining, where they facilitate the cracking of hydrocarbons and other chemical transformations. These reactions are essential for producing high-value products such as gasoline, diesel, and jet fuel.
Sulfur Removal: Vanadium catalysts, derived from are used in desulfurization processes, which remove sulfur from fuels and gases to reduce environmental pollution.