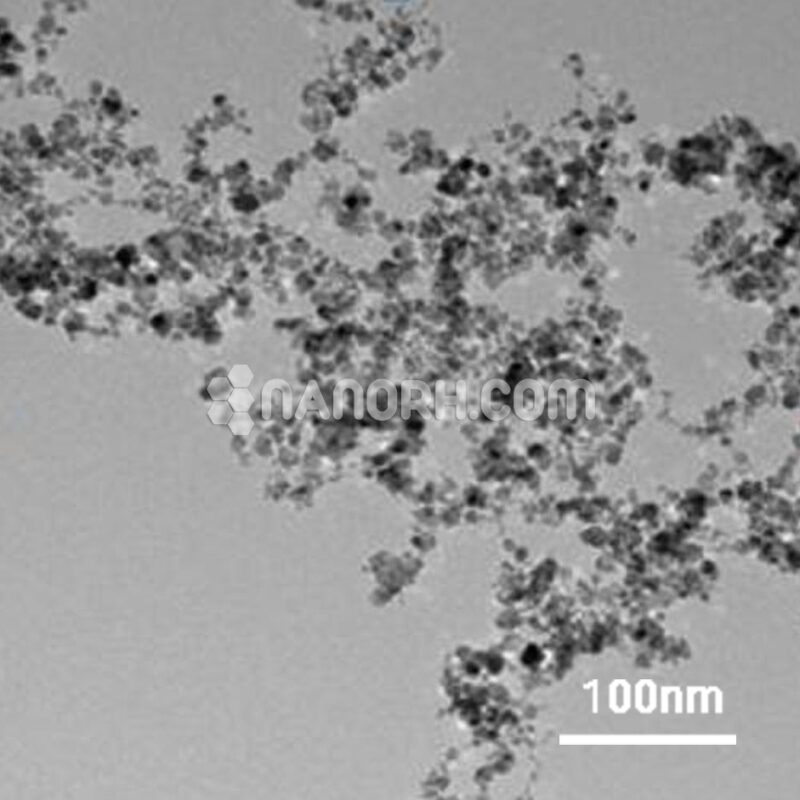
| Tin Sulfide Powder | |
| Product No | NRE-11253 |
| CAS | 1314-95-0 |
| Purity | 99.9% |
| Formula | SnS |
| APS | <40 µm (Can be Customized) |
| Color | Dark Brown |
| Molecular Weight | 150.78 g/mol |
| Density | 5.22 g/mL |
| Melting Point | 882 °C |
| Boiling Point | 1230 ˚C |
Tin Sulfide Powder
Tin Sulfide powder is an important inorganic compound composed of tin (Sn) and sulfur (S), and it exists in two main forms: tin(II) sulfide (SnS) and tin(IV) sulfide (SnS₂). The most commonly studied and used form of tin sulfide is tin(II) sulfide (SnS), which is a semiconducting material with a layered structure. Tin sulfide typically appears as a brownish to black powder and is known for its versatile electronic properties, making it a valuable material for a wide range of industrial applications, especially in the fields of electronics, optical devices, and energy storage.
SnS is a member of the metal chalcogenide family, which consists of compounds formed by metals combined with chalcogen elements such as sulfur, selenium, and tellurium. Metal chalcogenides, including SnS, are studied for their ability to exhibit a wide range of optical, electronic, and thermoelectric properties, making them useful in applications such as solar cells, photodetectors, and battery technologies.
Structure and Properties of Tin Sulfide (SnS)
Crystal Structure: Tin sulfide (SnS) crystallizes in an orthorhombic structure, which is characteristic of layered semiconducting materials. The layers in SnS are weakly bound by van der Waals forces, which allows for flexibility in terms of structure and potential for nanoscale applications. This layered structure can be exfoliated to produce thin films and nanomaterials.
Bandgap: SnS has a direct bandgap of around 1.3 eV, making it suitable for solar energy applications, particularly in thin-film solar cells. The bandgap allows SnS to absorb a significant portion of the solar spectrum, making it effective at converting light into electricity.
Thermal and Electrical Properties: Tin sulfide is also known for its low thermal conductivity and high electrical conductivity, which makes it efficient in thermoelectric and electronic devices. The low thermal conductivity helps SnS to retain heat, which is beneficial for thermoelectric applications where heat needs to be absorbed and converted into electricity.
Optical Properties: SnS exhibits strong optical absorption in the infrared and visible regions of the electromagnetic spectrum, making it a promising candidate for infrared sensors, photovoltaic devices, and photodetectors.