| Tantalum Hafnium Carbide Nanoparticles | |
| Product No | NRE-5215 |
| CAS | 71243-79-3 |
| Purity | 99.9% |
| Formula | TaC/HfC |
| APS | <100 nm (can be customized) |
| Color | Gray black |
| Molecular Weight | 962.34 g/mol |
| Density | NA |
| Melting Point | 3990 °C |
| Boiling Point | NA |
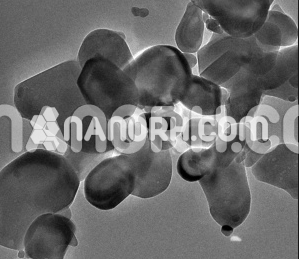
Tantalum Hafnium Carbide Nanoparticles
Introduction
Tantalum hafnium carbide nanoparticles these materials belong to the group of refractory ceramics and have garnered significant attention due to their exceptional properties, which include a high melting point, outstanding hardness, thermal conductivity, and chemical stability. The combination of tantalum and hafnium carbides results in a material with enhanced performance characteristics compared to individual carbides of either element alone, making TaHfC nanoparticles highly desirable in advanced technology applications.
Tantalum and hafnium are both transition metals that form strong, covalent bonds with carbon, leading to the formation of compounds that are not only thermally stable but also resistant to wear, oxidation, and other forms of degradation at extreme temperatures. When synthesized at the nanoparticle level, the unique properties of TaHfC are further enhanced by the increased surface area, which allows for more effective interactions in various applications, from high-performance coatings to catalytic processes.
Synthesis of TaHfC Nanoparticles
The preparation of TaHfC nanoparticles typically involves high-temperature processes, such as chemical vapor deposition (CVD), sol-gel methods, or ball milling, where fine powders of tantalum and hafnium precursors are combined with a carbon source (such as graphite or carbon black) to form the carbide nanoparticles. The size and morphology of the nanoparticles can be controlled by adjusting synthesis parameters such as temperature, pressure, and precursor concentration.
The controlled production of TaHfC nanoparticles results in a material with unique nanoscale properties, including a high surface area, small particle size, and increased reactivity compared to bulk forms of the material. This nanoparticulate form of TaHfC is of particular interest for applications where the material needs to interact at the molecular level, such as in catalysis, energy storage, and advanced coatings.