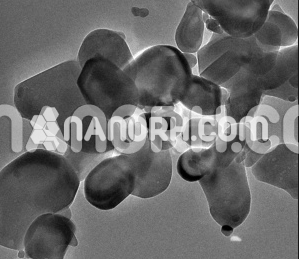
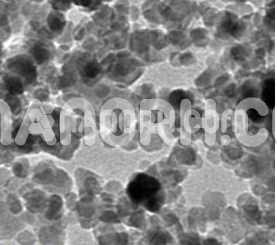
| Strontium Titanate Nanoparticles | |
| Product No | NRE-5211 |
| CAS No. | 12060-59-2 |
| Formula | SrTiO3 |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | Black |
| Molecular Weight | 183.49 g/mol |
| Density | 5.11 g/cm3 |
| Melting Point | 2,080 °C |
| Boiling Point | NA |
Strontium Titanate Nanoparticles
Strontium titanate nanoparticles have a wide range of applications in physics and chemistry: as highly polarizable nonlinear anode material, superconductors, semiconductors, substrates, photocatalysts, solid electrolytes, and solid oxide fuel cells (SOFCs). Its properties have been found to be highly dependent on particle size in the sub-100–200 nm range. For example, it has been shown that the photoluminescence intensity (PL) of SrTiO3 nanoparticles at room temperature increases with decreasing particle size. At the same time, the pronounced PL in single crystals is only observable at low temperatures. An increase in, for example, the band gap and lattice constant with decreasing nanoparticle size has been reported and attributed to the presence of surface dipoles. This makes particle size an important parameter for property modification and for designing materials with desirable properties. In general, SrTiO3 nanoparticles can be produced by physical (magnetron sputtering, laser ablation, etc.) and chemical processes. In this article, we would like to give a brief overview of the latest chemical preparation methods, discuss their main advantages and disadvantages, and compare some of the reported results with ours. In contrast to BaTiO3, little work has been published on the synthesis of SrTiO3 nanoparticles, although the chemical properties of Ba and Sr are quite similar and often the BaTiO3 fabrication routes can be applied to SrTiO3. A similar approach applies to PbTiO3 and partly to zirconates. However, in solutions under the same conditions, SrTiO3 nanoparticles seem to grow more than BaTiO3; this may be related to the fact that the crystal growth of BaTiO3 is more inhibited than in SrTiO3 due to the higher bonding strength and shielding effect of the solvent molecules in BaTiO3 compared to SrTiO3. This controls the growth rate of BaTiO3 relative to that of SrTiO3. It should be noted that the brief descriptions of nanoscale (Ba, Sr) TiO3 formulation methods presented in do not mention the resulting particle sizes. A detailed overview of liquid-phase syntheses is given, but covering all types of inorganic nanoparticles.