| OH Functionalized Short MWCNTs | |
| Product No | NRE-35006 |
| CAS No. | NA |
| Purity | Carbon nanotubes > 95wt% |
| Average Diameter | 50-80 nm |
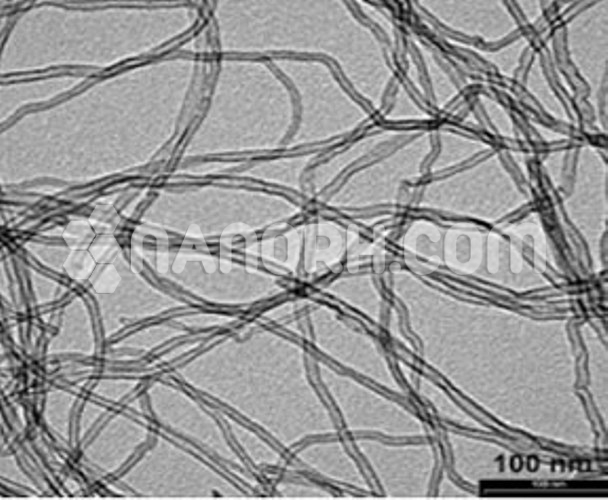
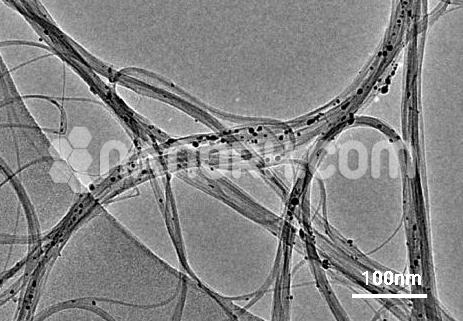
| Average Length | 0.5-2 um (TEM) |
| Special Surface Area(SSA) | >40m2/g(BET) |
| Tap Density | 0.18g/cm3 |
| True Density | 2.1g/cm3 |
| Electric Conductivity | > 100 S/cm |
OH Functionalized Short MWCNTs
Introduction
Multi-walled carbon nanotubes (MWCNTs) are cylindrical structures made of concentric graphene layers with exceptional properties such as high mechanical strength, electrical conductivity, and thermal stability. However, their application in various fields is often limited due to their tendency to agglomerate, low solubility, and poor interaction with other materials. To overcome these limitations, MWCNTs are commonly modified through functionalization, a process where chemical groups are introduced onto the surface of the nanotubes to enhance their properties.
One of the most effective methods of functionalization involves attaching hydroxyl groups (-OH) to the surface of MWCNTs. The introduction of hydroxyl groups significantly improves the dispersion and reactivity of MWCNTs, making them more suitable for various applications. These functionalized short MWCNTs, which have relatively small lengths compared to long MWCNTs, exhibit a higher surface-area-to-volume ratio, further enhancing their performance in various domains.
Features
Enhanced Dispersion:
The introduction of hydroxyl groups on the surface of MWCNTs makes them more hydrophilic and allows for better dispersion in water and other polar solvents. This reduces the tendency of MWCNTs to agglomerate and promotes their uniform distribution in composite materials.
Increased Surface Reactivity:
Hydroxyl groups provide polar functional sites that increase the reactivity of MWCNTs. These sites can engage in chemical bonding with other molecules, enabling further functionalization or interaction with a variety of materials.
Improved Biocompatibility:
OH-functionalized MWCNTs exhibit improved biocompatibility compared to unmodified MWCNTs. The hydroxyl groups enhance interactions with biological systems, reducing potential toxicity and making them suitable for applications in biomedicine, such as drug delivery and tissue engineering.
Increased Surface Area:
Short MWCNTs already possess a high surface-area-to-volume ratio. When functionalized with hydroxyl groups, this surface area is further optimized, allowing for more efficient adsorption and interaction with other materials, which is advantageous in applications like catalysis or sensing.