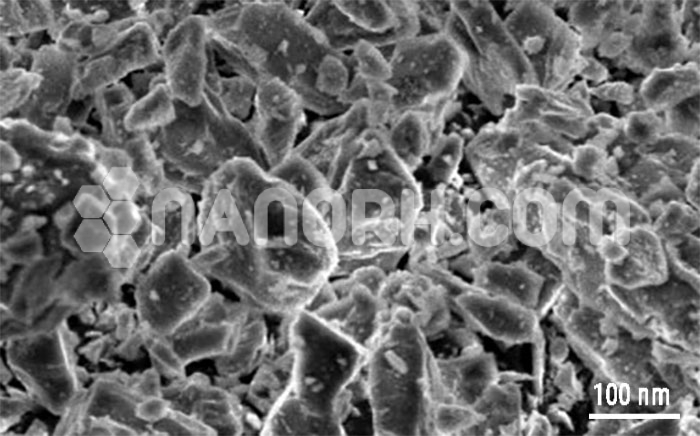
Nickel(II) Oxide Nanowires
Nickel(II) Oxide Nanowires are nanoscale wires with various applications including optical coatings, electronics, liquid crystals, transparent thin films and medical therapies. Silver nanowires are generally immediately available in most volumes. Elements produces to many standard grades when applicable…
| Nickel(II) Oxide Nanowires | |
| Product No | NRE-13008 |
| CAS No. | 1313-99-1 |
| Formula | NiO |
| Average diameter | 150-200nm |
| Average Length | up to 15um |
| Purity | 99.9% |
| Molecular Weight | 74.69 g/mol |
| Density | 6.67 g/cm3 |
| Melting Point | 1955 °C (3551 °F) |
| Boiling Point | NA |
Nickel(II) Oxide Nanowires are nanoscale wires with various applications including optical coatings, electronics, liquid crystals, transparent thin films, and medical therapies. Silver nanowires are generally immediately available in most volumes. Elements produce to many standard grades when applicable, including Mil Spec, ACS, Reagent and Technical Grade; Food, Agricultural and Pharmaceutical Grade.
Properties of Nickel(II) Oxide Nanowires
High Surface Area: NiO nanowires have a high surface-area-to-volume ratio, which enhances their reactivity and makes them excellent candidates for applications in catalysis, energy storage, and sensing.
Electrical Conductivity: Nickel oxide is a semiconductor, and NiO nanowires can exhibit both n-type and p-type conductivity depending on their synthesis method and processing conditions. This tunable electrical behavior makes NiO nanowires useful for a variety of electronic applications.
Magnetic Properties: NiO is antiferromagnetic at room temperature, meaning the magnetic moments of the nickel ions align oppositely, resulting in zero net magnetization. However, at the nanoscale, NiO nanowires can exhibit strong magnetic anisotropy, which makes them useful in magnetic sensors and spintronic devices.
Catalytic Activity: NiO is a highly active catalyst in oxidation reactions, including oxygen evolution and carbon monoxide oxidation. Its catalytic activity can be further enhanced when formed into nanowires due to their high surface area and ability to facilitate surface reactions.
Thermal Stability: NiO nanowires exhibit excellent thermal stability, making them suitable for use in high-temperature applications, such as thermal management systems and catalytic processes.
Chemical Reactivity: Nickel oxide nanowires can undergo redox reactions and can adsorb a variety of chemical species on their surface. This makes them useful for sensing applications and in environmental remediation processes.
Optical Properties: NiO nanowires exhibit some interesting optical properties, such as light absorption in the visible range and potential use in photocatalysis and photovoltaic devices.