| Nickel Selenide Nanoparticles | |
| Product No | NRE-5171 |
| CAS | 1314-05-2 |
| Purity | 99.9% |
| Formula | NiSe |
| APS | <100 nm (Can be Customized) |
| Color | NA |
| Molecular Weight | 137.65 g/mol |
| Density | 7.2 g/cm3 |
| Melting Point | NA |
| Boiling Point | NA |
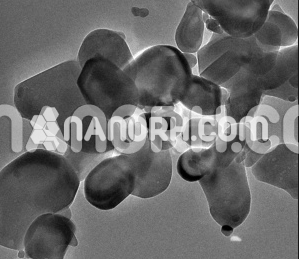
Nickel selenide Nanoparticles
These Nanoparticles are a p-type semiconductor with a mass energy gap of 2.0 eV (620 nm). Since it is a Pauli magnet, much interest has been directed to the synthesis of NiSe Nanoparticles. The Nanoparticles reported in this study was synthesized by reacting nickel chloride with selenium reduced with sodium borohydride. This method previously reported by us provides a robust and fast path for the formation of NiSe Nanoparticles. It has long been established that concentration has an effect on the resulting properties of nanomaterials. Several studies, in particular on CdS nanoparticles, have shown that anisotropic morphologies are favored at higher monomer concentrations and these determine different electronic spectra compared to isotropic morphologies. Hydrothermal syntheses of metal selenides with different reactant ratios were also performed and produced fairly large particles with unusual morphologies. Since nickel can exhibit different oxidation states, it is of particular interest to explore the preferred stoichiometries of nickel selenide in a controlled manner. The synthesis of nickel selenide with varying molar concentrations of nickel and selenium is reported. The optical properties of nickel selenide prepared with different reagent ratios are established.