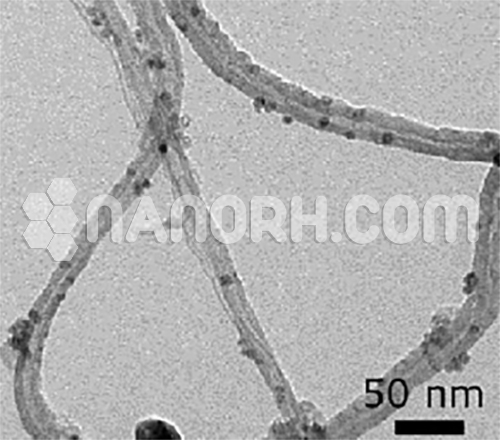
Nickel coated Carbon Nanotubes (Ni/CNT)
| Nickel coated Carbon Nanotubes | |
| Product No | NRE-42004 |
| CAS No. | 7440-02-0/308068-56-6 |
| Outer Diameter | 35-50 nm |
| Inner Diameter | 5-25nm |
| Average Length | 10-20um |
| True Density | 2.1 g/cm3 |
| Electric Conductivity | > 100 S/cm |
| Metal Percentage | 2-5% (can be Customized) |
Nickel coated Carbon Nanotubes – CNTs decorated with metal nanoparticles (NPs) like Silver nanoparticles, Gold nanoparticles, Nickel nanoparticles, copper nanoparticles, Magnesium nanoparticles, palladium nanoparticles, platinum nanoparticles, exhibit outstanding chemical activity due to their large active surface area and unique crystallographic surface structure.
Ni coated Carbon Nanotubes – Carbon nanotubes can be decorated with indirect and direct physicochemical and physical methods mainly with noble and transition metals.
Applications
Energy Storage and Conversion
Batteries: Nickel-coated CNTs are being explored for use in batteries, particularly in nickel-metal hydride (NiMH) batteries and other advanced battery technologies. The nickel layer enhances the electrical conductivity of CNTs, improving charge/discharge efficiency and the overall performance of the battery.
Supercapacitors: The combination of the high surface area of CNTs and the conductivity of nickel makes Ni-CNTs excellent candidates for use in supercapacitors, which store and release energy quickly. They can help improve the energy storage capacity and efficiency of supercapacitors.
Fuel Cells: Nickel-coated CNTs are used in fuel cell technology, where they serve as electrodes or catalysts. Nickel is a valuable material for catalyzing the hydrogen oxidation reaction (HOR) and the oxygen reduction reaction (ORR) in fuel cells. The CNTs provide high surface area and stability, enhancing the performance of the fuel cell.
Catalysis and Chemical Reactions
Catalysts in Hydrogenation: Nickel is widely used as a catalyst in hydrogenation reactions (e.g., in the chemical and petrochemical industries). Nickel-coated CNTs enhance catalytic efficiency due to the high surface area of CNTs, which allow for a greater number of active sites for catalytic reactions.
Carbon Nanotube-Based Catalysts: Ni-CNTs are used in electrocatalysis, such as in the development of catalysts for fuel cells and water splitting. The catalytic activity of nickel combined with the high surface area and conductivity of CNTs makes this composite a highly effective material for energy conversion processes.
CO2 Reduction: Nickel-coated CNTs have also shown promise as catalysts in the reduction of carbon dioxide (CO2) to useful products, such as methane or ethanol, as part of efforts to combat climate change and develop sustainable energy sources.