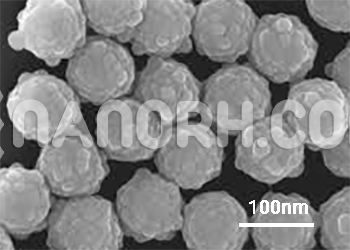
| Iron-Nickel Silica Core-Shell Nanoparticles | |
| Product No | NRE-16049 |
| CAS No. | 7439-89-6/7440-02-0/7631-86-9 |
| Formula | Fe Ni/SiO2 |
| APS | <100nm (can be customized) |
| Shape | Spherical |
| Purity | 99.9% |
| Core | Iron-Nickel (FeNi) |
| Shell | Silica |
| Appearance | Grey Powder |
| Boiling Point | NA |
Iron-Nickel Silica Core-Shell Nanoparticles
Applications
Magnetic Applications
Magnetic Separation and Purification: The magnetic properties of the Fe-Ni core make these core-shell nanoparticles ideal for use in magnetic separation processes. In environmental and industrial applications, these nanoparticles can be used to remove heavy metals, toxins, or organic pollutants from water or air by attracting and separating them from the medium. The silica shell provides chemical stability and protects the magnetic core from corrosion or oxidation, making these nanoparticles reusable.
Magnetic Targeted Drug Delivery: The magnetic core of Fe-Ni@SiO₂ nanoparticles enables the controlled delivery of drugs to specific targeted areas in the body, such as tumors or inflammatory tissues. By applying an external magnetic field, these nanoparticles can be guided to the desired site, where the silica shell can be functionalized with drugs for localized drug release, improving the efficacy and minimizing side effects of treatments, particularly in cancer therapy.
Magnetic Resonance Imaging (MRI): The magnetic properties of the iron-nickel core can be exploited in MRI as contrast agents, enhancing imaging resolution. The silica shell helps to prevent the nanoparticles from aggregating and ensures their biocompatibility, making them effective for in vivo imaging and diagnostic applications.
Catalysis
Catalysis in Hydrogenation Reactions: Iron-nickel alloys are known for their high catalytic activity in various reactions, particularly hydrogenation reactions. Fe-Ni@SiO₂ nanoparticles can act as efficient catalysts for the hydrogenation of unsaturated compounds, such as alkenes, alkynes, and aromatics. The silica shell not only provides chemical protection but also allows for easy recovery and reuse of the catalyst, making it cost-effective for industrial-scale processes.
Fischer-Tropsch Synthesis: The Fe-Ni alloy core of Fe-Ni@SiO₂ nanoparticles can also be used in the Fischer-Tropsch synthesis process, which is a key method for converting syngas (a mixture of carbon monoxide and hydrogen) into liquid hydrocarbons like synthetic fuels. The combination of iron and nickel in the core enhances the catalytic efficiency of this process, while the silica shell helps to stabilize the particles and improve their longevity in industrial applications.
Electrocatalysis: Fe-Ni@SiO₂ nanoparticles are also being studied for use in electrocatalytic reactions, such as the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER), which are important for fuel cells and hydrogen production. The metallic core (Fe-Ni) provides high electrocatalytic activity, while the silica shell can enhance stability and durability under the harsh conditions typical of electrocatalytic processes.
Energy Storage and Conversion
Supercapacitors: Fe-Ni@SiO₂ nanoparticles have shown promise as electrode materials for supercapacitors due to their high surface area, magnetic properties, and electrochemical activity. The iron-nickel core provides high capacitance, while the silica shell can enhance the long-term stability of the supercapacitor by preventing corrosion or oxidation of the active materials.
Lithium-Ion Batteries (LIBs): These core-shell nanoparticles have potential applications as anode materials in lithium-ion batteries. The iron-nickel core exhibits high electrochemical capacity, while the silica shell can act as a structural support to maintain the integrity of the anode during cycling. This helps improve the cycle stability and charging capacity of the battery.
Photovoltaics: Fe-Ni@SiO₂ nanoparticles can also be explored for photovoltaic applications in solar cells. The catalytic activity of the iron-nickel core can enhance the efficiency of photoelectrochemical cells by facilitating charge transfer, while the silica shell can help improve stability and durability under sunlight exposure.