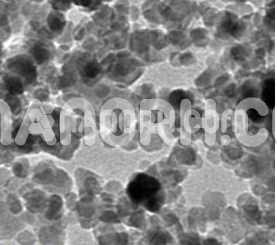
| Gallium Nitride Nanoparticles | |
| Product No | NRE-5086 |
| CAS | 25617-97-4 |
| Purity | 99.9% |
| Formula | GaN |
| APS | <100 nm (can be customized) |
| Color | Yellow |
| Molecular Weight | 83.73 g/mol |
| Density | 6.1 g/cm3 |
| Melting Point | 1600 °C |
| Boiling Point | NA |
Gallium Nitride Nanoparticles
Gallium Nitride nanoparticles is a wide-bandgap semiconductor material composed of gallium (Ga) and nitrogen (N). Gallium nitride nanoparticles are a form of GaN that have been reduced to the nanoscale, which offers distinct properties due to their increased surface area and size-dependent effects. GaN nanoparticles exhibit high thermal stability, optical properties, electrical conductivity, and high resistance to radiation and corrosion, making them ideal for a variety of advanced technological applications.
Properties
Wide Bandgap Semiconductor:
GaN is a wide-bandgap semiconductor with a bandgap of 3.4 eV, which makes it suitable for use in high-power and high-frequency applications. The wide bandgap allows GaN nanoparticles to function in high-temperature environments and under high-voltage conditions without significant degradation.
Luminescence and Photonic Properties:
Gallium nitride nanoparticles exhibit strong blue and ultraviolet (UV) luminescence, making them highly valuable in optical applications such as LEDs, lasers, and displays. When doped with certain elements, such as europium (Eu) or cerium (Ce), GaN nanoparticles can also emit light at specific wavelengths, which makes them suitable for bioimaging and sensing applications.
High Thermal Stability:
GaN nanoparticles exhibit excellent thermal conductivity and stability. This property is crucial for devices that need to operate at high temperatures and in demanding conditions, such as in aerospace, automotive, and power electronics applications.
Synthesis of Gallium Nitride Nanoparticles
Chemical Vapor Deposition (CVD):
CVD is a widely used method for synthesizing high-quality GaN nanoparticles. This process involves reacting gaseous gallium precursors (e.g., gallium chloride (GaCl₃) or trimethylgallium (TMGa)) with nitrogen sources (e.g., ammonia (NH₃) or nitrogen gas (N₂)) under controlled temperature and pressure conditions.