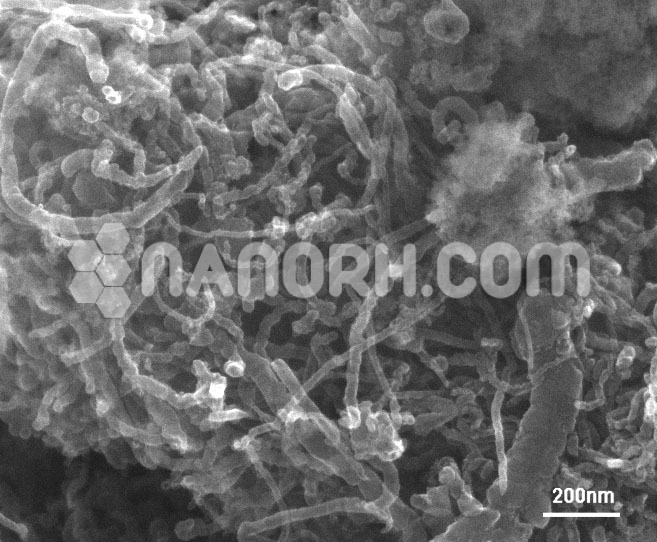
Fullerene C60, 99%
Fullerenes are poorly soluble in most solvents and are usually solubilized with aromatic solvents such as toluene, chlorobenzene, or non-aromatic solvents such as carbon disulfide. Pure fullerene solution is usually purple, the concentration is dark purple…
Fullerene C60, 99%
| Product No | NRE-41003 |
| CAS | 99685-96-8 |
| Purity | >99% |
| Melting Point | >280 °C |
| Morphology | Spherical |
| Flash Point | > 94 °C |
| Density | 1.6 g/cm³ at 20 °C |
| Molecular Formula | C60 |
| Molecular Weight | 720.64 g/mol |
| Form | Crystalline powder |
| Orbital energy | HOMO 6.1-6.2 eV |
| Orbital energy | UMO 4.5 eV |
| Reactivity | Non Reactive/ Non Soluble |
| Stability | Completely Stable |
| Solubility | Soluble in organic solvents |
Fullerenes C60 Solubility
Fullerenes C60 solubility are a fascinating class of carbon molecules that exhibit a variety of unique properties. Among these, often referred to as buckminsterfullerene, is the most well-known and widely studied member of the fullerene family. This molecule consists of 60 carbon atoms arranged in a spherical structure, resembling a soccer ball, where the carbon atoms are connected in a combination of pentagons and hexagons.
C60 was discovered in 1985 by scientists Robert Curl, Harold Kroto, and Richard Smalley, who were awarded the Nobel Prize in Chemistry in 1996 for their groundbreaking work on the discovery and structure of fullerenes.
Molecular Structure and Properties
C60 is made up of 60 carbon atoms bonded together in a closed, spherical shape. The structure can be described as a truncated icosahedron, consisting of 12 pentagonal and 20 hexagonal faces. This structure is highly symmetric and resembles a soccer ball, which is why it is sometimes referred to as the buckyball.
The carbon-carbon bonds in are sp2 hybridized, similar to the bonds found in graphite, and each carbon atom is bonded to three other carbon atoms. The arrangement of atoms within C60 imparts various properties, such as high chemical stability, electronic properties (which make it an excellent electron acceptor), and the ability to withstand high pressures and temperatures.
While C60’s unique structure makes it a highly interesting and versatile molecule, its solubility behavior is a critical aspect of its practical use. The solubility of C60 influences how it can be processed, incorporated into various materials, and applied in scientific research and industrial applications.