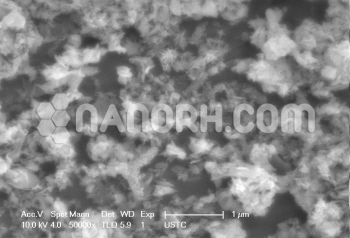
| Copper Silver Coated Nanopowder | |
| Product No | NRE-16093 |
| CAS No. | 7440-22-4/7440-50-8 |
| Formula | CuAg |
| APS | <100nm (can be customized) |
| Color | Gray Brown |
| Purity | 99.9% |
| Molecular Weight | 171.41 g/mol |
| Density | 9.49 g/cm3 |
| Melting Point | 1061.78°C |
| Boiling Point | 2262°C |
Copper Silver Coated Nanopowder
Silver is a chemical element with the symbol Ag (from the Latin Argentum, derived from pr proto-Indo-European Pr: “bright” or “white”) and the atomic number 47. A soft, white, and shiny transition metal shows the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. The metal is found in the earth’s crust in the pure and free elementary form (“native silver”), as an alloy with gold and other metals, and in minerals such as argentite and chloraguita. Most of the silver is produced as a by-product of the refining of copper, gold, lead, and zinc.
Silver has long been considered a precious metal. The silver metal is used in many coins, sometimes together with gold: while it is more abundant than gold, it is much less abundant than the native metal. Its purity is typically measured by thousandth; a 94% purity alloy is described as “0.940 fine”. As one of the seven metals of antiquity, silver has had a lasting role in most human cultures.
In addition to money and as a means of investment (coins and ingots), silver is used in solar panels, water filtration, jewelry, ornaments, tableware, and utensils of great value (hence the term silver), in electrical contacts and drivers, in specialized applications. Mirrors, window coverings, in catalysis of chemical reactions, such as stains of colored glass, and in specialized confectionery. Its compounds are used in photographic and radiographic films diluted solutions of silver nitrate and other silver compounds are used as disinfectants and microbicides (oligodynamic effect), these are added to bandages and dressings, catheters, and other medical instruments.