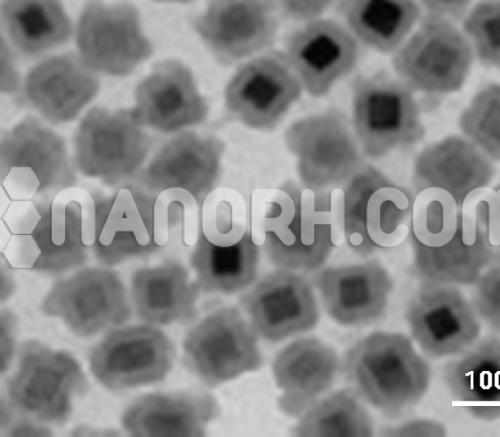
| Cobalt Monoxide Nanoparticles | |
| Product No | NRE-29003 |
| CAS No. | 1307-96-6 |
| Formula | CoO |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | Black |
| Molecular Weight | 74.9326 g/mol |
| Density | 6.44 g/cm³ |
| Melting Point | 1935 °C |
| Boiling Point | NA |
Cobalt Monoxide Nanoparticles
CoO nanoparticles have been obtained mainly from the thermal decomposition of organic cobalt salts in the presence of protective agents in organic solvents with long hydrocarbon chains. The synthetic methods are quite complex and involve expensive and toxic organic cobalt salts as precursors, limiting both their synthesis and their large-scale application. Additionally, capping agents are difficult to remove and tend to mask active sites and decrease electroactivity. Another approach to obtain CoO nanoparticles is the pyrolysis of Co3O4 in an inert atmosphere, although severe agglomeration of CoO nanoparticles occurs during phase transformation as the required temperature is typically up to 900° C for bulk materials. It is known that the temperature required for the phase transformation of nano dimensional particles decreases significantly compared to that of the bulk material. Activated from this point, we propose to synthesize CoO nanoparticles by pyrolysis of NP CoOx with well controlled nano dimensions. Moreover, NP CoO with well-controlled dimensions are obtained by manipulating the dimensions of NP CoOx. For the first time, the impact of cobalt monoxide particle size on ORR activity is investigated. It should be mentioned that carbon often