| Calcium Sulfide Nanoparticles | |
| Product No | NRE-5050 |
| CAS No. | 20548-54-3 |
| Formula | CaS |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | Off White |
| Molecular Weight | 72.143 g/mol |
| Density | 2.59g/cm3 |
| Melting Point | 2525°C |
| Boiling Point | NA |
Calcium Sulfide Nanoparticles
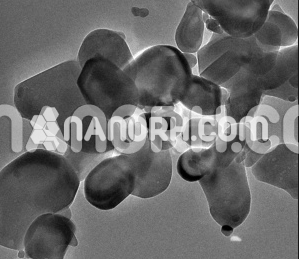
Calcium sulfide nanoparticles exists naturally as the mineral lapis calcareus, but it is primarily synthesized in the laboratory or industrial settings by combining calcium oxide (CaO) with hydrogen sulfide (H₂S) or by direct sulfiding of calcium metal. When calcium sulfide is reduced to nanoparticle form, the material exhibits remarkable optical, electrical, and chemical properties. The nanoscale particles typically range from 1 to 100 nanometers and show improved surface reactivity, photoluminescence, and thermal stability compared to the bulk material.
Due to the high reactivity of sulfur in its elemental state and the specific properties that arise at the nanoscale, calcium sulfide nanoparticles have potential applications in several advanced technological fields.
Properties
High Surface Area:
As with many nanomaterials, calcium sulfide nanoparticles exhibit a significant increase in surface area relative to their bulk counterpart. This results in enhanced reactivity, making the nanoparticles effective in chemical processes and catalysis, where surface interactions are crucial.
Photoluminescence:
One of the key features of is their ability to exhibit photoluminescence. This means that when the particles are exposed to light, they can absorb energy and re-emit it as visible light. The color of the emitted light can vary depending on the size of the nanoparticles, which allows for tuning of their optical properties.
Thermal Stability:
possess good thermal stability, which allows them to withstand high temperatures without significant degradation. This makes them suitable for high-performance applications where temperature resilience is important.