| Barium Nitride Nanoparticles | |
| Product No | NRE-5021 |
| CAS | 12047-79-9 |
| Purity | 99.9% |
| Formula | Ba3N2 |
| APS | <100 nm (can be customized) |
| Color | Orange-yellow |
| Molecular Weight | 439.99 g/mol |
| Density | 4.78 g/cm3 |
| Melting Point | NA |
| Boiling Point | NA |
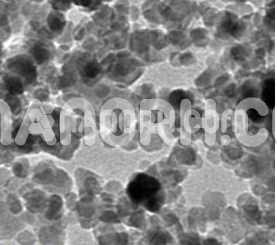
Barium Nitride Nanoparticles
Barium Nitride nanoparticles is an inorganic compound composed of barium and nitrogen, typically found in its bulk form as a crystalline material with an ionic structure. However, when reduced to nanoparticle form, barium nitride undergoes significant changes in its properties, such as increased surface area, reactivity, and enhanced physical and chemical characteristics.
Applications
Energy Storage and Conversion
Supercapacitors and Batteries: Barium nitride nanoparticles are studied for use in energy storage devices such as supercapacitors and batteries due to their high electrical conductivity and ion mobility. These devices benefit from the high surface area and electronic properties of the nanoparticles, which allow for fast charge/discharge cycles and high energy storage capacity.
Fuel Cells: Barium nitride nanoparticles are also considered for use in fuel cells, where they could serve as electrode materials or catalysts. Their high stability and reactivity could enhance the performance of hydrogen fuel cells, improving the efficiency of energy conversion.
Catalysis and Chemical Reactions
Catalytic Reactions: Barium nitride nanoparticles are explored for use in nanocatalysis applications. The high surface area and reactivity of the nanoparticles make them effective in hydrogenation, oxidation, and CO₂ reduction reactions. They are particularly valuable in green chemistry applications, where they can help reduce energy consumption and improve reaction efficiency.
CO₂ Reduction: Barium nitride nanoparticles are also being studied for their potential in carbon capture and storage (CCS) technologies. Their surface reactivity can be utilized to enhance the absorption and conversion of CO₂, contributing to carbon-neutral energy processes.
Electronics and Sensors
Electronic Devices: Ba3N2 due to their electrical conductivity, can be used in advanced electronic devices such as semiconductors, diodes, and transistors. Their high electron mobility and stability at the nanoscale make them useful for high-performance electronic circuits.
Gas Sensors: The high reactivity and surface area of make them ideal candidates for gas sensing applications. They can be used in sensors to detect gases like nitrogen dioxide (NO₂), ammonia (NH₃), and hydrogen (H₂). These sensors have applications in industrial monitoring, environmental sensing, and safety devices.
Photodetectors: Barium nitride nanoparticles can be used in photodetectors and optical sensors, as they exhibit optical properties that are enhanced at the nanoscale. They can be incorporated into devices that detect light in specific wavelengths, such as infrared or ultraviolet light, for communication and sensing applications.