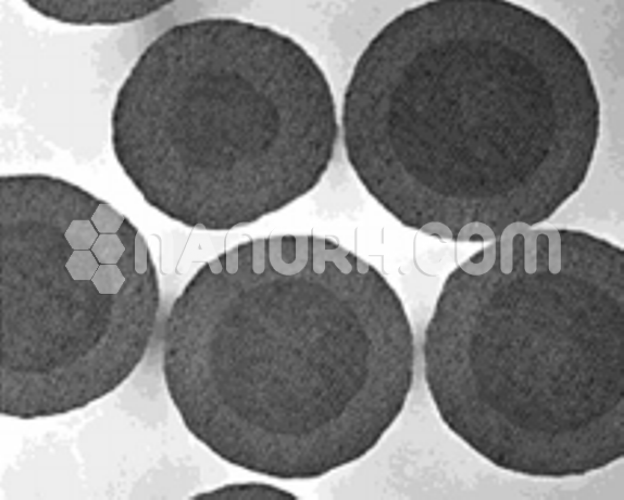
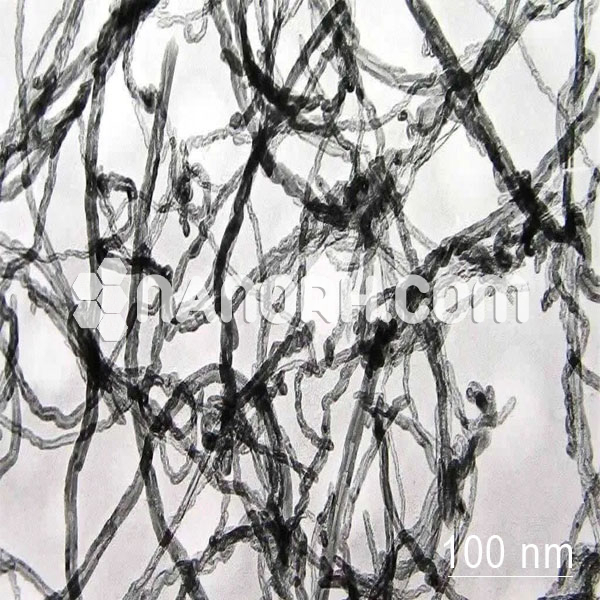
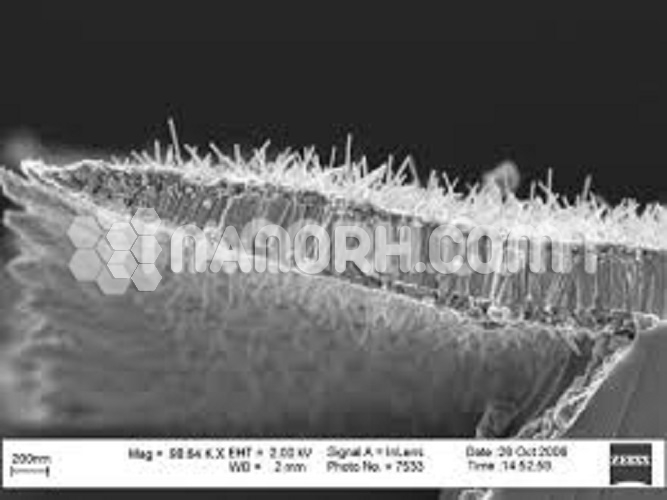
Calcium Oxide/Iron Oxide Core Shell Nanoparticles (CaO/Fe2O3, 99.9%, APS: 80-100nm, Metal Oxide Core)
| Calcium Oxide/Iron Oxide Core Shell Nanoparticles | |
| Product No | NRE-16017 |
| CAS No. | NA |
| Formula | CaO/Fe2O3 |
| APS | <100nm (can be customized) |
| Shape | Spherical |
| Purity | 99.9% |
| Core | Calcium Oxide |
| Shell | Iron Oxide |
| Appearance | Powder |
| Boiling Point | NA |
Calcium Oxide/Iron Oxide Core-Shell Nanoparticles
Applications
Catalysis
CO2_22 Capture and Carbonate Formation: CaO is widely used for CO2_22 capture because of its ability to absorb CO2_22 at high temperatures. The Fe2_22O3_33 shell can improve the reactivity and regeneration efficiency of the CaO core, making the core-shell nanoparticles useful in carbon capture and storage technologies.
Acid-Base Catalysis: The basic properties of CaO combined with the oxidative capabilities of Fe2_22O3_33 make these nanoparticles effective in acid-base catalysis, such as in biodiesel production or esterification reactions.
Oxidation Reactions: The iron oxide shell can enhance the catalytic activity in oxidation reactions, such as the degradation of organic pollutants or the oxidation of CO to CO2_22. The magnetic properties of Fe2_22O3_33 also facilitate easy separation and reuse of the catalysts.
Environmental Remediation
Pollutant Degradation: The CaO/Fe2_22O3_33 core-shell nanoparticles can be used for the photocatalytic degradation of pollutants, particularly in water treatment. The iron oxide shell acts as a catalyst under visible light, while the CaO core can interact with acidic pollutants, neutralizing them.
Heavy Metal Removal: These nanoparticles are also effective in removing heavy metals like lead and mercury from wastewater. The CaO core can interact with these metals, while the iron oxide shell facilitates the magnetic separation of the particles.
Magnetic Separation and Recycling
The Fe2_22O3_33 shell provides strong magnetic properties, enabling the core-shell nanoparticles to be easily separated from reaction mixtures using a magnetic field. This is particularly useful in catalyst recycling, where the same nanoparticles can be reused in multiple cycles without losing their efficiency.
Energy Storage
Thermal Energy Storage: The high thermal stability of CaO makes it suitable for thermal energy storage applications, where it can absorb heat during the day and release it when needed. The iron oxide shell can improve the material’s stability and performance in these applications, particularly in high-temperature environments.
Supercapacitors and Batteries: The core-shell nanoparticles can also be used in supercapacitors or batteries as electrodes, with the iron oxide shell providing additional electronic conductivity and the CaO core enhancing the ion storage capacity.
Magnetic Resonance Imaging (MRI)
The magnetic properties of the Fe2_22O3_33 shell make these nanoparticles potential candidates for use in MRI contrast agents. The CaO core could also improve biocompatibility, making the nanoparticles suitable for medical imaging and diagnostic purposes.