| Cobalt Sulphide Nanoparticles | |
| Product No | NRE-5066 |
| CAS No. | 1317-42-6 |
| Formula | CoxSy |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | Gray |
| Molecular Weight | 90.9982 g/mol |
| Density | 5.45 g/cm³ |
| Melting Point | 1195 °C |
| Boiling Point | NA |
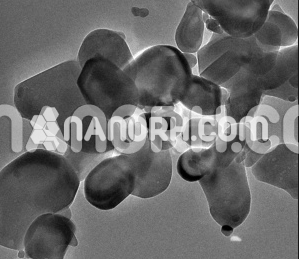
Cobalt Sulphide Nanoparticles
Introduction
Cobalt sulfide nanoparticles is a binary compound composed of cobalt and sulfur, existing in several stoichiometric forms, including Co₃S₄, CoS, and Co₄S₃. Cobalt sulfide nanoparticles, specifically at the nanoscale, exhibit unique physical and chemical properties such as high surface area, high reactivity, and tunable electrical, magnetic, and catalytic characteristics. These properties make cobalt sulfide nanoparticles highly promising for a wide range of applications in fields like energy storage, catalysis, environmental remediation, and magnetic materials.
Applications
Catalysis:
Hydrogen Evolution Reaction (HER): Cobalt sulfide nanoparticles are effective electrocatalysts for the hydrogen evolution reaction (HER) in water splitting processes. This is essential for the generation of hydrogen gas as a clean energy source. Their high catalytic activity and good conductivity make them efficient in producing hydrogen from water using renewable energy sources.
Oxygen Evolution Reaction (OER): Cobalt sulfide nanoparticles are also used as catalysts in the oxygen evolution reaction (OER), which is important for generating oxygen in water splitting and other electrochemical reactions. Their catalytic efficiency improves energy conversion processes, which is beneficial for renewable energy technologies such as solar-to-hydrogen systems.
Organic Reactions: In addition to water splitting, cobalt sulfide nanoparticles have shown catalytic activity in various organic transformations, including hydrogenation and oxidation reactions, which are important for industries like pharmaceuticals, petrochemicals, and fine chemicals.
Energy Storage:
Supercapacitors: Cobalt sulfide nanoparticles are used in supercapacitors due to their high surface area and good electrical conductivity. Supercapacitors store energy by accumulating charge on their surface, and cobalt sulfide nanoparticles improve the energy density, cycling stability, and charge-discharge efficiency of these devices. Supercapacitors find applications in electric vehicles, backup power, and renewable energy systems.
Batteries: Cobalt sulfide nanoparticles are also explored as materials for battery electrodes, particularly in lithium-ion and sodium-ion batteries. They improve capacity, charge/discharge rates, and cycle stability.