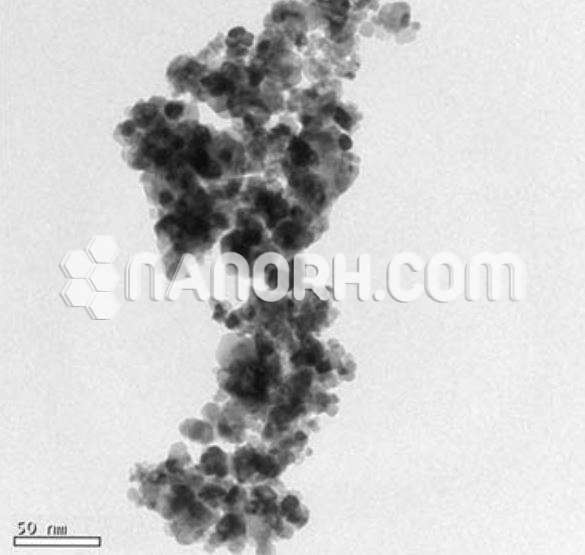
| Tin Oxide Nanopowder | |
| Product No | NRE-7004 |
| CAS No. | 18282-10-5 |
| Formula | SnO2 |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | White |
| Molecular Weight | 150.71g/mol |
| Density | 6.95 g/cm3 |
| Melting Point | 1630 °C |
| Boiling Point | 1800-1900 °C |
Tin Oxide Nanopowder
Introduction:
Tin Oxide (SnO₂) Nanopowder is a nanomaterial that has garnered significant attention due to its unique physical and chemical properties, including its high surface area, excellent conductivity, and catalytic capabilities. Tin oxide is an important n-type semiconductor, which means that it is electron-rich and can conduct electricity through the movement of electrons. When reduced to the nanoscale (typically ranging from 10 to 100 nanometers), SnO₂ exhibits significantly enhanced properties compared to its bulk counterpart, making it useful for a variety of applications in electronics, sensors, energy storage, and environmental technologies.
Properties
High Surface Area: Nanoparticles have a much larger surface area-to-volume ratio compared to bulk materials, which increases their reactivity and catalytic efficiency. This makes SnO₂ nanopowder ideal for use in sensors and catalytic applications.
Semiconducting Nature: SnO₂ is a wide-bandgap semiconductor, which means it has a large energy difference between its conduction band and valence band. This property makes SnO₂ useful in electronic applications, especially in devices like sensors and transistors.
Optical Properties: SnO₂ exhibits optical transparency in the visible region, and its high refractive index is useful in applications such as coatings and sensors. Its ability to be transparent while conducting electricity makes it valuable in optoelectronics.
Catalytic Activity: Tin oxide has good catalytic properties, especially for oxidation reactions. This makes it useful in environmental applications, such as air and water purification, as well as in energy production processes like fuel cells.
Synthesis
There are several methods for synthesizing tin oxide nanoparticles, each offering distinct advantages in terms of control over particle size, morphology, and purity. Some common synthesis techniques include:
Sol-Gel Method: This chemical method involves the transformation of a liquid precursor into a gel that then forms nanoparticles. It offers good control over the size and uniformity of SnO₂ nanoparticles.
Hydrothermal Synthesis: A method that involves high-temperature and high-pressure conditions in the presence of water or aqueous solutions to produce SnO₂ nanoparticles. This technique helps in obtaining high-quality, crystalline nanopowders.