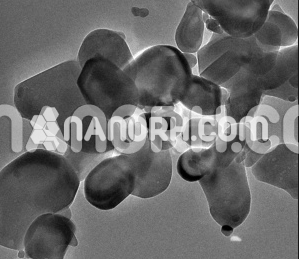
| Titanium Diboride Nanopowder | |
| Product No | NRE-5232 |
| CAS No. | 12045-63-5 |
| Formula | TiB2 |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | Dark Grey |
| Molecular Weight | 69.489 g/mol |
| Density | 4.52 /cm3 |
| Melting Point | 3230 °C |
| Boiling Point | NA |
Titanium Diboride Nanopowder
Titanium Diboride Nanopowder combines a high melting point ,high hardness, high modulus of elasticity , low resistivity, high thermal conductivity, good chemical stability and corrosion resistance in aggressive gaseous and liquid media, and low density, which allows it to find effective applications in various areas of modern engineering and industry. Recent years have seen a considerable increase of interest in titanium diboride and related compounds because they have been used as basic components for producing nanomaterials with various and excellent physicochemical, mechanical, and other properties, differing significantly from the properties of their microcrystalline analogs. TiB2 nanopowder can be prepared via the thermolysis of titanium borohydride derivatives at a temperature The TiB2 Nanopowder prepared according to this scheme in the form of powder or film is X-ray amorphous and crystallizes in vacuum after annealing between 1173 and 1273 K. However, the described process takes a considerable time and is multistep, so the resultant TiB2 Nanopowder is contaminated with appreciable amounts of carbon and oxygen. TiB2 Nanopowder can also be prepared through mechanochemical interaction of titanium(III) chloride with lithium hydride and lithium borohydride in a highenergy mill according to the reaction scheme obtained TiB2 Nanopowder with a crystallite size of a few nanometers via ~873-K heat treatment of a LiBH4 + TiO2 mixture preactivated in a high-energy mill. In addition, Titanium Diboride Nanopowder can be prepared through mechanochemical interaction of microcrystalline titanium and boron in a high-energy mill. Moreover, titanium diboride nanopowder can be obtained by reacting sodium borohydride with TiCl4 at elevated temperatures and pressures or by reacting Ti with BBr3 in the presence of sodium at 673 K and by a carbothermic process according to the reaction scheme