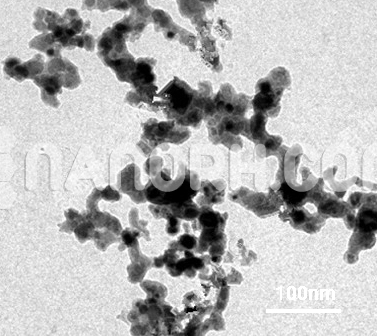
Copper Oxide Nanorods (CuO Nanorods, 99.5+%, Width:15-35nm, Length: 60-140nm)
Presentation to air, simple to ingest dampness, however does not create water mixes. It is solvent in nitric corrosive. At the point when warmed to over 1200 oC, nano-cobalt oxide will be separated into sub-cobalt oxide. In the hydrogen fire, nano-cobalt oxide is warmed to 900 oC, it will be changed into metal cobalt. Cobalt(II,III) oxide is substance compound with the equation Co3O4
| Copper Oxide Nanorods | |
| Product No | NRE-12010 |
| CAS No. | 1317-38-0 |
| Formula | CuO |
| Average diameter | 30-50nm |
| Average Length | up to 500nm |
| Purity | 99.9% |
| Molecular Weight | 79.5454 g/mol |
| Density | 6.31 g/cm³ |
| Melting Point | 1,326 °C |
| Boiling Point | 2,000 °C |
Copper Oxide Nanorods (CuO Nanorods, 99.5+%, Width:15-35nm, Length: 60-140nm) Application:
Insoluble in water. break down gradually in liquor or smelling salts arrangement. The solvent in weakening acids, NH4Cl, (NH4) 2CO3, potassium cyanide arrangement. Under high temperature, copper oxide meets with hydrogen or carbon monoxide, can reestablish copper metal. Nano-copper oxide is a broadly utilized material. It has been connected to the impetus, superconducting materials, thermoelectric materials, detecting materials, glass, pottery, and different fields. What’s more, the nano-copper oxide can be utilized as a rocket fuel ignition impetus. It not exclusively can essentially enhance the homogeneous charge consuming rate, bring down weight file, yet additionally can better execute as the impetus for the AP composite fuel. More utilize, for example, Ceramic resistors, Gas sensors, Magnetic capacity media, Near-infrared tilters, Photoconductive and photothermal applications, Semiconductors, Solar vitality change, Catalysts, High-tech superconductors……Cupric oxide is utilized as a shade in earthenware production to deliver blue, red, and green (and here and there dark, pink, or dark) coats. It is additionally used to create cuprammonium hydroxide arrangements, used to make rayon. It is likewise infrequently utilized as a dietary supplement in creatures, against copper lack. Copper(II) oxide has an application as a p-sort semiconductor since it has a restricted band hole of 1.2 eV. It is a grating used to clean optical hardware. Cupric oxide can be utilized to deliver dry cell batteries. It has likewise been utilized as a part of wet cell batteries as the cathode, with lithium as an anode, and dioxane blended with lithium perchlorate as the electrolyte. Copper(II) oxide can be utilized to create other copper salts. It is likewise utilized when welding with copper compounds…