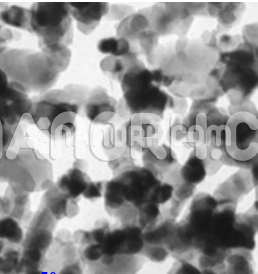
| Copper Nitride Nanoparticles | |
| Product No | NRE-5072 |
| CAS | 1308-80-1 |
| Purity | 99.9% |
| Formula | Cu3N |
| APS | <100 nm (can be customized) |
| Color | Gray |
| Molecular Weight | 204.6 g/mol |
| Density | 5.84 g/cm3 |
| Melting Point | NA |
| Boiling Point | NA |
Copper Nitride Nanoparticles
Copper nitride nanoparticles are a class of copper-based compounds that are characterized by the presence of nitrogen in their crystal structure. Copper nitride typically exists in the form of a copper(I) nitride (Cu₃N), which consists of copper in the +1 oxidation state and nitrogen atoms. These nanoparticles have attracted significant interest in materials science due to their unique electronic, optical, and catalytic properties, making them suitable for a wide range of applications across electronics, energy, and environmental technologies.
Synthesis
Copper nitride nanoparticles are typically synthesized using one of the following methods:
Chemical Vapor Deposition (CVD): This technique involves the reaction of copper precursors (such as copper vapor or copper salts) with nitrogen-containing gases (e.g., ammonia or nitrogen gas) at elevated temperatures. The process produces copper nitride films or nanoparticles with controlled morphology.
Ball Milling: Copper nitride can also be synthesized by grinding copper powder in the presence of nitrogen or ammonia gas under high-energy ball milling conditions. This method is simple and scalable, and it often results in nanoparticles with good dispersion and uniformity.
Solvothermal or Hydrothermal Methods: In this method, copper salts (like copper chloride or copper sulfate) are reacted with nitrogen sources (like ammonia or hydrazine) in a high-pressure, high-temperature environment.