| Lead Bromide Nanoparticles | |
| Product No | NRE-5125 |
| CAS | 10031-22-8 |
| Purity | 99.9% |
| Molecular Formula | PbBr2 |
| Molecular Weight | 367.01 g/mol |
| Color | White |
| Density | 6.66 g/cm3 |
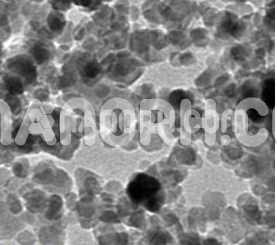
| APS | <100 nm (can be customized) |
| Melting Point | 370.6° C |
| Boiling Point | 916° C |
Lead Bromide Nanoparticles
Introduction
Lead bromide (PbBr₂) is an inorganic compound composed of lead (Pb) and bromine (Br), forming a crystalline salt with a chemical formula of PbBr₂. It is typically a white or colorless solid that is highly soluble in water and can be synthesized into nanoparticles with a variety of unique properties that differ from the bulk material. When reduced to the nanoscale, PbBr₂ nanoparticles exhibit an increase in surface area, reactivity, and can often show quantum effects that enhance their functionality in various applications.
Properties
Quantum Size Effects:
When PbBr₂ is reduced to the nanoscale, it exhibits quantum confinement effects, which result in altered electronic and optical properties. These effects can lead to significant changes in the band gap, absorption, and emission spectra of PbBr₂ nanoparticles compared to their bulk counterparts.
Optical Properties:
PbBr₂ nanoparticles are known for their photoactivity, making them useful in a range of optical applications. They exhibit a wide absorption spectrum and can emit fluorescence or phosphorescence under certain conditions, which makes them attractive for use in photoelectric and luminescent devices.
High Stability and Chemical Reactivity:
PbBr₂ is relatively stable at room temperature, but in its nanoparticle form, it can be more reactive due to its larger surface area. This enhances its potential for use in catalysis and other chemical applications where increased reactivity is desirable.
High Solubility:
PbBr₂ is highly soluble in water, which makes PbBr₂ nanoparticles particularly useful in applications that involve aqueous systems or where dissolution and interaction with other materials is required.
Toxicity Considerations:
Like many lead-based compounds, PbBr₂ nanoparticles are toxic, and handling requires caution. There is ongoing research to mitigate the environmental and health risks associated with lead-based nanoparticles while maximizing their utility in industrial and scientific applications.