| Chromium Sulfide Nanoparticles | |
| Product No | NRE-5063 |
| CAS | 12018-22-3 |
| Purity | 99.9% |
| Formula | Cr2S3 |
| APS | <100 nm (Can be Customized) |
| Color | Brown to black |
| Molecular Weight | 200.19 g/mol |
| Density | 3.77g/cm3 |
| Melting Point | 1,350° C |
| Boiling Point | NA |
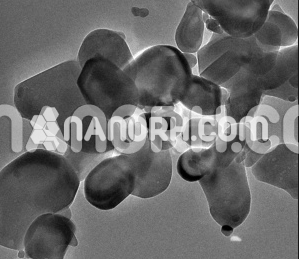
Chromium Sulfide Nanoparticles
Chromium sulfide nanoparticles is an inorganic compound formed by the combination of chromium and sulfur, typically existing in various stoichiometries, with Cr₂S₃ being the most common. When reduced to the nanoscale, chromium sulfide exhibits unique properties, including high surface area, increased chemical reactivity, and enhanced electronic and optical behaviors, making it an interesting material for a variety of advanced applications.
Chromium sulfide nanoparticles (Cr₂S₃ NPs) are typically synthesized through methods such as chemical vapor deposition (CVD), hydrothermal synthesis, sol-gel processes, or chemical reduction techniques.
Applications
Catalysis:
Chromium sulfide nanoparticles have been studied for their catalytic properties, particularly in reactions such as hydrogenation, oxidation, and dehydrogenation. Their high surface area and reactivity make them efficient catalysts or catalyst supports in the production of chemicals, fuels, and fine chemicals, as well as in environmental applications such as the degradation of pollutants.
Energy Storage:
Due to their conductive properties, are being explored for use in batteries and supercapacitors. They can be used as electrode materials or conductive additives, improving the energy storage capacity, charge-discharge rates, and cycle stability of these devices. Cr₂S₃ can also be incorporated into composite materials to enhance the performance of energy storage systems.
Photocatalysis:
Chromium sulfide nanoparticles have potential applications in photocatalysis, where they can be used to facilitate reactions driven by light. This could be applied in environmental remediation, such as water purification or air pollution control, by breaking down organic pollutants when exposed to light. Their photocatalytic properties are enhanced by their nanoscale dimensions, allowing for more efficient absorption of light and catalysis.