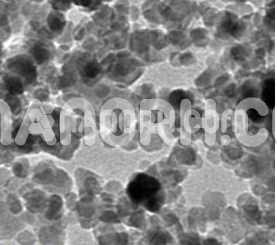
| Ferric Silicide Nanoparticles | |
| Product No | NRE-5081 |
| CAS No. | 12022-99-0 |
| Formula | FeSi2 |
| Density | 4.74 g/cm3 |
| APS | <100 nm (Can be Customized) |
| Purity | 99.9% |
| Form | Powder |
| Molecular Weight | 112.016 g/mol |
| Certificate Of Analysis | |
| Fe | 49.8% |
| Si | 50.1% |
| C | 0.02% |
| S | 0.03% |
| Fe | 0.02% |
| O | 0.01% |
| Sn | 0.01% |
Ferric Silicide Nanoparticles
Ferric silicide nanoparticles are a type of iron silicide compound consisting of iron (Fe) and silicon (Si) in a specific stoichiometric ratio. These nanoparticles are typically composed of iron in the +3 oxidation state (Fe³⁺) bonded with silicon atoms, forming a silicide structure that can display unique electrical, magnetic, and chemical properties. combine the beneficial features of iron (e.g., magnetic properties and catalytic activity) with those of silicon (e.g., semiconductor properties and structural stability).
Ferric silicide nanoparticles are generally synthesized through high-temperature methods or chemical vapor deposition (CVD), which result in a material with high surface area and reactive properties due to their nanoscale size. Their unique combination of properties makes suitable for a wide range of applications, particularly in electronics, magnetic materials, catalysis, and energy storage.
Properties
Magnetic Properties:
Iron-based compounds, including ferric silicide, typically exhibit magnetic properties due to the presence of iron. These properties can include superparamagnetism (a form of magnetism where nanoparticles can be magnetized and demagnetized with an applied magnetic field) or ferromagnetism depending on the nanoparticle size and morphology. can be utilized in magnetic sensors, data storage devices, and biomagnetic applications.
Electrical Conductivity:
Ferric silicide nanoparticles can have a semiconducting nature due to the silicon component. This makes them suitable for use in electronic devices, solar cells, and field-effect transistors. Their electrical properties can be further engineered by controlling particle size and doping with various elements.
Catalytic Activity:
As an iron-based material, ferric silicide nanoparticles exhibit catalytic properties that can be utilized in various chemical reactions. These nanoparticles can act as catalysts for hydrogenation, dehydrogenation, and CO₂ reduction reactions, making them useful in green chemistry and energy conversion applications.