| Sodium Fluoride Nanoparticles | |
| Product No | NRE-5206 |
| CAS No. | 7681-49-4 |
| Formula | NaF |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | White |
| Molecular Weight | 41.99 g/mol |
| Density | 2.558 g/cm³ |
| Melting Point | 993 °C |
| Boiling Point | 1,704 °C |
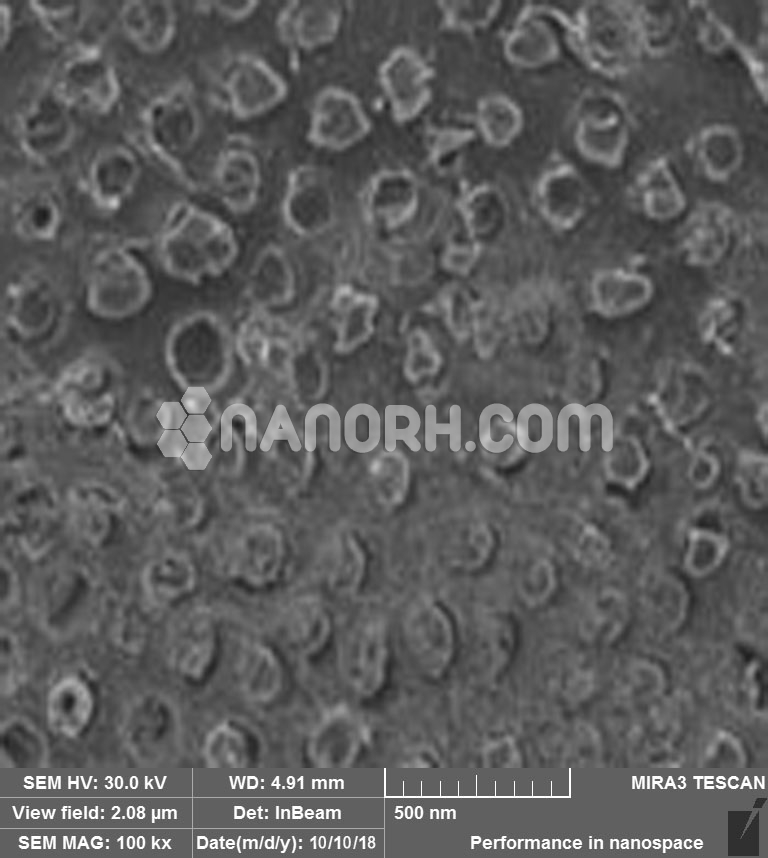
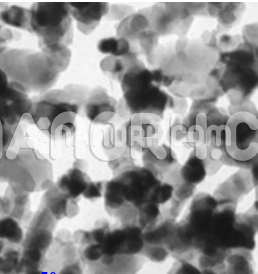
Sodium Fluoride Nanoparticles
Introduction to Sodium Fluoride (NaF) Nanoparticles
Sodium fluoride (NaF) is an inorganic compound composed of when reduced to the nanoscale, NaF forms nanoparticles (NaF NPs), which exhibit distinct physical, chemical, and biological properties compared to their bulk form. These nanoparticles have a high surface area to volume ratio, leading to enhanced reactivity, solubility, and biological activity, making them suitable for a variety of industrial, environmental, and medical applications.
Sodium fluoride is widely known for its use in dental care, particularly in toothpaste and mouth rinses, to help prevent tooth decay. However, when in nanoparticle form, NaF exhibits additional properties that are leveraged in various advanced fields, such as materials science, energy storage, catalysis, and environmental applications.
Properties of Sodium Fluoride Nanoparticles:
High Reactivity: Due to their small size, NaF nanoparticles exhibit higher reactivity than bulk NaF. This makes them suitable for catalysis, sensing, and other chemical reactions.
Enhanced Solubility: NaF nanoparticles can dissolve more easily in solutions, enhancing their effectiveness in applications like water treatment and chemical reactions.
Fluoride Release: NaF nanoparticles are capable of controlled release of fluoride ions, which is beneficial in medical and dental applications.
Biocompatibility: NaF nanoparticles are considered safe for specific medical and environmental uses, particularly when used in controlled concentrations.